Greeting

Toshio Shimizu, MD, PhD
Professor, Department of New Experimental Therapeutics
Director, Early Phase 1 Drug Development Service
Kansai Medical University Hospital
In order to provide cancer patients with access to the latest anti-cancer new drugs in the shortest and fastest time possible, our department is the first department across university hospital in Japan to specialize in early phase 1 oncology clinical trials, and we are keenly aware of the great responsibility that comes with this, and we are also very much aware of the importance of our mission. Currently, in Japan, facilities for conducting early phase FIH (first-in-human) clinical trials for new cancer drugs are limited to a few advanced cancer specialized institutions, mainly in the Tokyo metropolitan area. In order to eliminate drug loss and drug lags in the field of cancer treatment in Japan, we aim to be an innovative access point for clinical trials in Asia, providing cancer patients in West Japan with new cancer drugs that have only been developed overseas as quickly as possible, and making as many treatment options available as possible. Our main mission is to promote the early development of new cancer drugs (mainly phase I trials including FIH phase 1), and Kansai Medical University Hospital has a specialized outpatient clinic and inpatient ward for phase 1 clinical trials in the Department of New Experimental Therapeutics and Early Phase 1 Drug Development Service.
Kansai Medical University (KMU) will celebrate its 100th anniversary in 2028. At the same time, we are also working hard to become an international hub for the development of new cancer drugs in Asia, and to become a “Five-Stars” Oncology Early Phase 1 Center of Excellence.
We also welcome young doctors who can become the next generation of leaders, who share our understanding of the importance of developing new cancer drugs, and who have an international perspective and want to work with us to shape the future of new drug development in Japan.

Advantages
-

Rapid Phase 1 Site Activation in West Japan
-

Dedicated and Predominant Early Phase 1 Drug Development Service
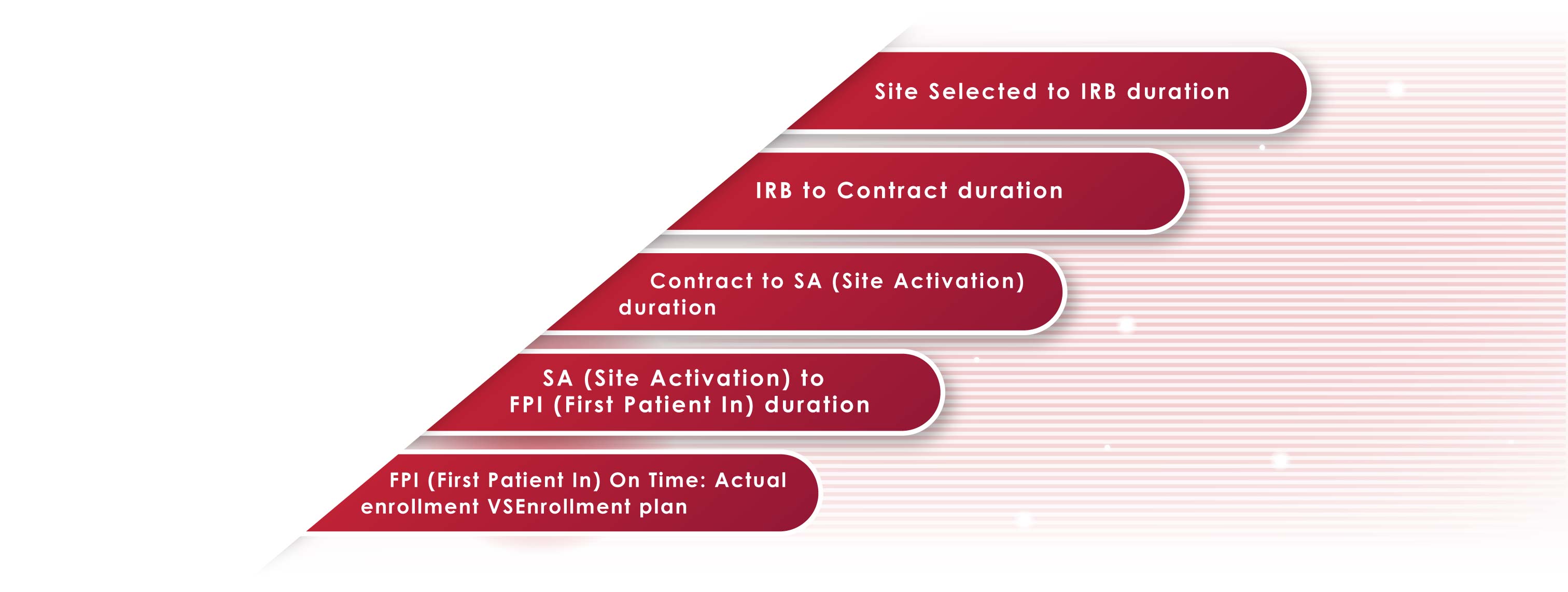
We especially
focus on
strengthening the
following key milestones
Mission and Goals
To provide advanced solid tumor patients in West Japan with the
earliest opportunities to access the latest drugs against a
specific cancer or cancer mutation
-
To provide advanced solid tumor patients in West Japan with the earliest opportunities to access the latest drugs against a specific cancer or cancer mutation
-
To advance the research and development of new treatments by determining the optimal safe doses, paving the way for mid-late phase studies including phase II and phase III

-
To facilitate the rapid evaluation of promising agents using advanced clinical trial designs, which target a specific genetic mutation across a range of tumors from different and diverse cancer types

-
To educate and train next generation clinical fellows

-
Experience
Kansai Medical University (KMU) was established as Osaka Women’ s Medical College in 1928 and KMU has 6 affiliated medical facilities in Osaka Japan. KMU Main Hospital with the latest facilities in Japan, opened in January 2006. It started as an advanced treatment hospital; KMU Hospital is a 3-minute walk with extremely conveniently accessible from Hirakata-shi Station on the Keihan Railways Line only 20 mins from both Osaka and Kyoto downtown urban area and it has facilities with a total floor space of 773397.7 sq/ft (71,851m2) on premises of 340268.7 sq/ft (31,612m2) with the waterfront area of the Yodogawa Riverside Park in Osaka behind it. In terms of our medical care system, we have been actively promoting the development of centers where multiple departments cooperate to provide medical care especially in cancer treatment and oncology drug development. At KMUH, we are currently conducting more than 200 clinical trials in all phases of research for many types of cancer. Of those, the Early Drug Development (EDD) Service, Department of New Experimental Therapeutics has been launched since November 2024 as an extremely dedicated and predominant early phase 1 drug development service.
-
Large Patient Pool
Many phase I trials require a certain number of cancer patients. As a high-volume cancer center, KMUH can fulfill recruitment objectives for many disease types, including rare cancers. Our expanded comprehensive care facilities at regional sites throughout the area including robust collaboration with large number of key cancer hospitals as well as university hospitals in West Japan are more convenient for participants and allow for faster study start-up and recruitment.

